Updated Protoplast preparations for gene transfection (11/30/09)
Protoplast isolation protocol is based on the method developed by Sheen and colleagues (Sheen, J. 2002, A transient expression assay using Arabidopsis mesophyll protoplasts.). The protocol establsihed for our lab can be downloaded from here.
Materials
- razor blade(single edge carbon steel, Electron Microscopy Sciences, Cat.#71950)
- 100 μm nylon mesh (cell strainer 100 μm, BD Biosciences, Cat.#352360)
- Digestion buffer
- 1%(w/v) cellulase "onozuka"R10 (Karlan Research Products Corp. or Yakult Pharmaceutical industry co.,ltd.)
0.25%(w/v) macerozyme R10 (Karlan Research Products Corp. or Yakult Pharmaceutical industry co.,ltd)
0.4 M mannitol
20 mM MES (pH 5.7)
20 mM KCl
10 mM CaCl2
Preparation
- Add 100 mg of cellulase R10 and 25 mg of macerozyme R10 into a 50 ml tube.
- Add 10 ml of buffer containing 0.4 M mannitol, 20 mM MES (pH 5.7), 20 mM KCl, 10 mM CaCl2 into the tube.
- Vortex the tube briefly, and shake in orbit at room temperature for few min (2-5 min).
*The digestion solution will be clear (light brown).
**No heat treatment and No filtration
- W5 buffer
- 154 mM NaCl
125 mM CaCl2
5 mM KCl
2 mM MES (pH 5.7) - MMg solution
- 0.4 M mannitol
15 mM MgCl2
4 mM MES (pH 5.7)
Protocols
- About one-month old Arabidopsis leaves (0.5 - 1.0 g) are cut into 1-2 mm strips with a razor blade on a copy paper.
- The leaf strips are transferred to 10 ml of digestion buffer in a Petri dish.
- The dish is then vacuum for 30 min at room temperature to permeate the buffer into the stripped leaves.
- The dish is placed in the dark for 6 hours at room temperature (25 °C).
- 10 ml of W5 buffer are added to the Petri dish.
- Filtere the protoplast suspension into a 50 ml tube with a 100 μm nylon mesh.
- Collect the protoplasts by centrifugation at 100×g for 3 min.
- Wash two times in 10 ml of W5 buffer through consecutive resuspension and centrifugation at 100×g for 3 min.
- Resuspend the protoplasts in 10 ml of W5 solution and are kept at 4 °C for 30 min.
- Collect the protoplasts by centrifugation at 100×g for 3 min.
- Wash in 5 ml of MMg solution.
- Resuspend the protoplast in 5 - 10 ml of the MMg solution to count the numbers in the suspension with a haematocytometer.
 |
 |
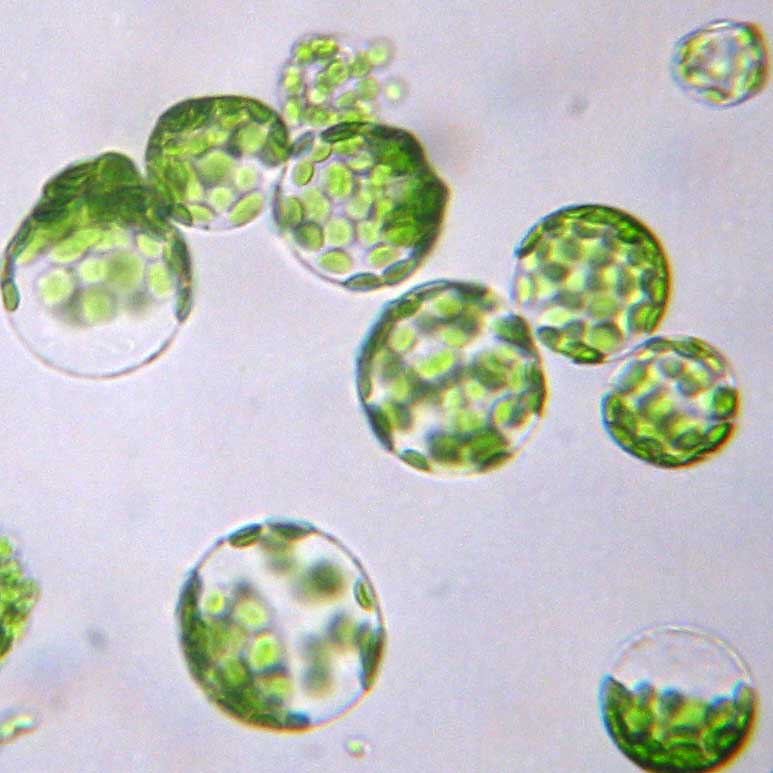 |
Pre-digestion |
Post-digestion |
Protoplast |